Lub xeev peb-dimensional ntawm cov kua dej yog qhov nyuaj rau kev kawm, tab sis ntau tau kawm los ntawm kev tshuaj xyuas cov qauv ntawm cov dej khov nab kuab. Plaub tus neeg nyob sib ze hydrogen-interacting oxygen atoms nyob ntawm qhov chaw ntawm tetrahedron (tetra=plaub, hedron=dav hlau). Qhov nruab nrab lub zog yuav tsum tau ua kom tawg zoo li daim ntawv cog lus hauv dej khov yog kwv yees li ntawm 23 kJ / mol-1.

Lub peev xwm ntawm cov dej molecules los tsim cov xov tooj ntawm hydrogen chains, nrog rau lub zog muab, tsim kom muaj qhov sib txawv ntawm qhov sib txawv. Thaum nws melts, nws yog tuav los ntawm cov kua dej, cov qauv ntawm cov uas tsis sib xws. Feem ntau ntawm cov hydrogen bonds yog distorted. Nws siv lub zog loj hauv daim ntawv ntawm cov cua sov kom tawg cov iav siv lead ua ntawm hydrogen-bonded ice.
Txoj kev zoo li dej khov (Ih)
Ntau tus neeg nyob hauv xav tsis thoob li cas cov dej khov siv lead ua lattice muaj. Tsim nyogNws yuav tsum raug sau tseg tias qhov ntom ntawm cov tshuaj feem ntau nce thaum khov, thaum molecular txav qeeb thiab ntom ntom ntom ntom ntom ntom ntom ntom. Qhov ceev ntawm cov dej kuj nce ntxiv thaum nws txias mus rau qhov siab tshaj plaws ntawm 4 ° C (277K). Tom qab ntawd, thaum kub poob qis dua tus nqi no, nws nthuav.
Qhov kev nce no yog vim qhov tsim ntawm qhov qhib, hydrogen-bonded ice crystal nrog nws cov lattice thiab qis dua ntom ntom, nyob rau hauv txhua qhov dej molecule yog rigidly khi los ntawm cov ntsiab lus saum toj no thiab plaub lwm qhov tseem ceeb, thaum txav ceev txaus rau muaj ntau pawg. Txij li thaum qhov kev ua no tshwm sim, cov kua dej khov ntawm sab saum toj mus rau hauv qab. Qhov no muaj cov txiaj ntsig tseem ceeb hauv kev noj qab haus huv, vim tias cov txheej txheem dej khov ntawm lub pas dej insulates nyob deb ntawm huab cua txias. Tsis tas li ntawd, ob qho khoom ntxiv ntawm dej muaj feem xyuam rau nws cov yam ntxwv hydrogen: tshwj xeeb kub thiab evaporation.
Kev piav qhia ntawm cov qauv
Thawj qhov ntsuas yog qhov xav tau los nce qhov kub ntawm 1 gram ntawm cov khoom los ntawm 1 ° C. Kev nce qib ntawm cov dej yuav tsum muaj cov cua kub ntau vim tias txhua lub molecule koom nrog ntau cov hydrogen bonds uas yuav tsum tau tawg kom lub zog kinetic nce. Los ntawm txoj kev, qhov ntau ntawm H 2 O nyob rau hauv cov hlwb thiab cov ntaub so ntswg ntawm tag nrho cov loj multicellular kab mob txhais tau hais tias qhov kub thiab txias nyob rau hauv lub hlwb yog txo. Qhov no feature yog qhov tseem ceeb, txij li tus nqi ntawm feem ntau cov tshuaj biochemicalrhiab heev.
Qhov kub ntawm vaporization ntawm dej kuj tseem siab dua li ntawm ntau lwm cov kua. Cov cua kub ntau yuav tsum tau hloov lub cev no mus rau hauv cov pa roj, vim tias cov ntawv cog lus hydrogen yuav tsum tau tawg kom cov dej molecules kom txav ntawm ib leeg thiab nkag mus rau theem ntawd. Lub cev hloov tau yog cov dipoles mus tas li thiab tuaj yeem cuam tshuam nrog lwm cov khoom sib xws thiab cov uas ionize thiab yaj.
Lwm cov tshuaj hais los saum toj no tuaj yeem tiv tau tsuas yog muaj polarity. Nws yog qhov sib xyaw ua ke uas koom nrog hauv cov qauv ntawm cov khoom no. Tsis tas li ntawd, nws tuaj yeem ua ke ib ncig ntawm cov khoom no tsim los ntawm electrolytes, kom cov pa oxygen tsis zoo ntawm cov dej molecules yog taw qhia rau cations, thiab cov ions zoo thiab hydrogen atoms yog taw qhia rau cov anions.
Hauv cov khib nyiab, raws li txoj cai, molecular crystal lattices thiab atomic sawv daws yuav tsim. Qhov ntawd yog, yog tias iodine tsim nyob rau hauv txoj kev uas nws muaj I 2, , ces nyob rau hauv cov pa roj carbon dioxide, uas yog, nyob rau hauv qhuav dej khov, CO2 molecules yog. nyob rau ntawm cov kab lattice nodes . Thaum sib cuam tshuam nrog cov khoom zoo sib xws, cov dej khov muaj ionic siv lead ua lattice. Graphite, piv txwv li, uas muaj cov qauv atomic raws li cov pa roj carbon, tsis tuaj yeem hloov tau, ib yam li pob zeb diamond.
Yuav ua li cas thaum lub pob zeb ntawm lub rooj ntsev yaj hauv dej: cov polar molecules tau nyiam rau cov ntsiab lus hauv cov khoom siv lead ua, uas ua rau tsim cov khoom zoo sib xws ntawm sodium thiab chloride ntawm nws qhov chaw, ua rau lub cev no.yog dislocated ntawm ib leeg, thiab nws pib yaj. Los ntawm no nws tuaj yeem pom tias cov dej khov muaj cov iav siv lead ua nrog ionic bonding. Txhua yaj Na + nyiam qhov tsis zoo kawg ntawm ob peb cov dej molecules, thaum txhua yaj Cl - nyiam qhov zoo kawg. Lub plhaub nyob ib puag ncig txhua ion hu ua txoj kev khiav tawm thiab feem ntau muaj ob peb txheej ntawm cov kuab tshuaj.
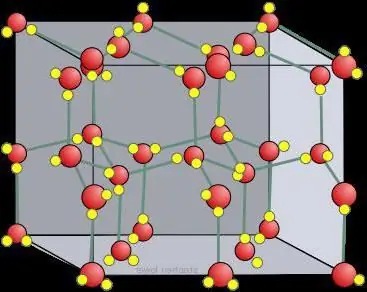
Qhia dej khov siv lead ua lattice
Variables los yog ib qho ion ncig los ntawm cov ntsiab lus tau hais tias yog sulfated. Thaum cov kuab tshuaj yog dej, cov khoom no yog hydrated. Yog li, ib qho polar molecule zoo li raug daws los ntawm cov ntsiab lus ntawm lub cev ua kua. Hauv cov dej khov qhuav, hom siv lead ua lattice tsim atomic bonds hauv lub xeev ntawm kev sib sau ua ke, uas tsis hloov pauv. Lwm qhov yog cov dej khov crystalline (dej khov). Ionic organic tebchaw xws li carboxylase thiab protonated amines yuav tsum soluble hauv hydroxyl thiab carbonyl pawg. Cov khoom uas muaj nyob rau hauv cov qauv no txav ntawm cov molecules, thiab lawv cov polar systems tsim hydrogen bonds nrog lub cev no.
Tau kawg, tus naj npawb ntawm cov pab pawg kawg hauv cov molecule cuam tshuam rau nws cov solubility, uas tseem nyob ntawm cov tshuaj tiv thaiv ntawm ntau cov qauv hauv lub caij: piv txwv li, ib-, ob- thiab peb-carbon cawv yog miscible. nrog dej, tab sis cov hydrocarbons loj dua nrog ib qho hydroxyl compounds tsawg dua hauv cov kua.
Hexagonal Ih zoo ib yam liatomic siv lead ua lattice. Rau cov dej khov thiab tag nrho cov daus ntuj hauv ntiaj teb, nws zoo nkaus li zoo li no. Qhov no yog pov thawj los ntawm symmetry ntawm crystal lattice ntawm cov dej khov, zus los ntawm dej vapor (uas yog, snowflakes). Nws yog nyob rau hauv qhov chaw pab pawg P 63 / hli los ntawm 194; D 6h, Laue class 6/mm; zoo ib yam li β-, uas muaj ntau yam ntawm 6 helical axis (kev sib hloov ncig ntxiv rau kev hloov ntawm nws). Nws muaj kev ncaj ncees qis dua cov qauv qis qis uas ua tau zoo (~ 1/2) lossis lub ntsej muag nruab nrab (~ 3/4).
Piv rau cov dej khov zoo tib yam, cov siv lead ua lattice ntawm cov dej khov qhuav, khi los ntawm CO2 molecules, zoo li qub thiab hloov pauv tsuas yog thaum atoms lwj.
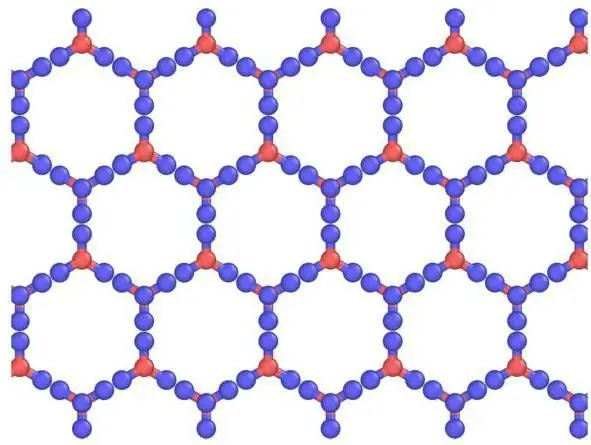
Kev piav qhia ntawm gratings thiab lawv cov ntsiab lus
Crystals tuaj yeem pom raws li cov qauv crystalline, uas muaj cov nplooj ntawv tso rau saum ib sab. Lub hydrogen daim ntawv cog lus yog txiav txim, thaum nyob rau hauv kev muaj tiag nws yog random, txij li thaum protons tuaj yeem txav ntawm cov dej (ice) molecules ntawm qhov kub siab tshaj li 5 K. Qhov tseeb, nws yuav zoo li cov protons coj zoo li quantum kua nyob rau hauv tas li tunneling ntws. Qhov no tau txhim kho los ntawm kev tawg ntawm cov neutrons, qhia lawv qhov sib txawv ntawm qhov sib txawv ntawm ib nrab ntawm cov pa atoms, qhia tias thaj chaw thiab kev sib koom ua ke. Ntawm no muaj qhov zoo sib xws ntawm cov dej khov nrog lub atomic, molecular crystal lattice.
Molecules muaj staggered kev npaj ntawm hydrogen sawnrog kev hwm rau nws peb tus neeg nyob ze hauv lub dav hlau. Lub caij thib plaub muaj kev sib koom ua ke ntawm hydrogen daim ntawv cog lus. Muaj qhov sib txawv me ntsis ntawm qhov zoo tshaj plaws hexagonal symmetry, vim tias chav tsev ntawm tes yog 0.3% luv dua nyob rau hauv cov kev taw qhia ntawm cov saw no. Tag nrho cov molecules muaj tib lub molecular ib puag ncig. Muaj qhov chaw txaus hauv txhua "lub thawv" los tuav cov khoom ntawm cov dej sib txuas. Txawm hais tias tsis yog feem ntau txiav txim siab, lawv tsis ntev los no tau raug kuaj pom zoo los ntawm neutron diffraction ntawm hmoov crystal lattice ntawm dej khov.
Hloov Tshuaj
Lub cev hexagonal muaj peb npaug cov ntsiab lus nrog cov kua thiab cov dej gaseous 0.01 ° C, 612 Pa, cov khoom siv - peb -21.985 ° C, 209.9 MPa, kaum ib thiab ob -199.8 ° C, 70 MPa, thiab - 34.7 ° C, 212.9 MPa. Lub dielectric tas li ntawm hexagonal ice yog 97.5.
Lub melting nkhaus ntawm lub ntsiab yog muab los ntawm MPa. Cov kev sib npaug ntawm lub xeev muaj, ntxiv rau lawv, qee qhov tsis sib xws yooj yim hais txog kev hloov pauv ntawm lub cev lub zog rau qhov kub thiab txias ntawm cov dej khov hexagonal thiab nws cov aqueous suspensions. Hardness fluctuates nrog degrees nce los ntawm los yog qis dua gypsum (≦2) ntawm 0 ° C rau feldspar (6 Mohs) ntawm -80 ° C, ib qho txawv txav loj hloov nyob rau hauv kiag hardness (> 24 zaug).
Lub hexagonal siv lead ua lattice ntawm cov dej khov ua rau hexagonal daim hlau thiab kab, qhov twg lub ntsej muag sab sauv thiab sab qis yog cov dav hlau basal {0 0 0 1} nrog ib qho enthalpy ntawm 5.57 μJ cm -2, thiab lwm qhov sib npaug yog hu ua ntu ntawm prism {1 0 -1 0} nrog 5, 94µJ cm -2. Qhov chaw thib ob {1 1 -2 0} nrog 6.90 ΜJ ˣ cm -2 tuaj yeem tsim raws cov dav hlau tsim los ntawm ob sab ntawm cov qauv.
Cov qauv zoo li no qhia tau hais tias qhov txo qis hauv thermal conductivity nrog kev nce siab (nrog rau cov dej khov nab kuab thiab amorphous ntawm qhov ntom ntom), tab sis txawv ntawm cov muaju feem ntau. Qhov no yog vim muaj kev hloov pauv hauv daim ntawv cog lus hydrogen, uas txo qhov kev sib hloov ceev ntawm lub suab hauv cov iav siv lead ua ntawm cov dej khov thiab dej.
Muaj txoj hauv kev piav qhia yuav ua li cas npaj cov qauv siv lead ua loj thiab txhua qhov dej khov uas xav tau. Nws yog assumed tias hydrogen daim ntawv cog lus nyob rau saum npoo ntawm lub hexagonal lub cev nyob rau hauv kev kawm yuav raug txiav txim ntau tshaj nyob rau hauv lub bulk system. Variational spectroscopy nrog theem-lattice zaus tiam tau pom tias muaj cov qauv asymmetry ntawm ob txheej txheej sab saud (L1 thiab L2) nyob rau hauv subsurface HO saw ntawm basal nto ntawm hexagonal ice. Cov saws hydrogen bonds nyob rau hauv lub sab sauv txheej ntawm lub hexagons (L1 O··· HO L2) muaj zog tshaj cov uas tau txais nyob rau hauv lub thib ob txheej mus rau lub sab sauv (L1 OH ··· O L2). Sib tham sib hexagonal ice qauv muaj.
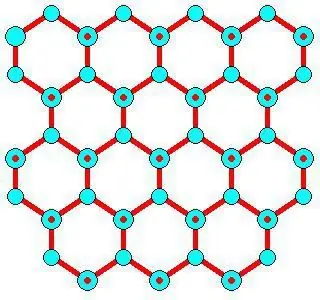
Kev tsim kho yam ntxwv
Tus naj npawb tsawg kawg ntawm cov dej molecules xav tau los ua dej khov yog kwv yees li 275 ± 25, raws li kev ua tiav icosahedral pawg ntawm 280. Kev tsim tshwm sim ntawm tus nqi ntawm 10 10 ntawm huab cua-dej interface thiab tsis nyob rau hauv tej dej. Kev loj hlob ntawm cov dej khov muaju yog nyob ntawm qhov sib txawv ntawm kev loj hlob ntawm ntau yamlub zog. Dej yuav tsum tau tiv thaiv los ntawm khov thaum khaws cia cov khoom lom, khoom noj thiab cov khoom nruab nrog cev.
Qhov no feem ntau ua tiav los ntawm kev ua kom txias sai, siv cov qauv me me thiab cov khoom siv cryoconservator, thiab ua kom lub siab ua kom cov dej khov thiab tiv thaiv kev puas tsuaj ntawm tes. Lub zog dawb ntawm cov dej khov / kua dej nce los ntawm ~ 30 mJ / m2 ntawm atmospheric siab mus rau 40 mJ / m -2ntawm 200 MPa, qhia vim li cas thiaj li tshwm sim.
Yuav ua li cas siv lead ua lattice yog yam ntxwv ntawm cov dej khov
Hloov pauv, lawv tuaj yeem loj hlob sai ntawm qhov chaw prism (S2), ntawm qhov tsis sib xws ntawm cov pas dej khov los yog kub ntxhov. Kev loj hlob los ntawm {1 1 -2 0} lub ntsej muag yog yam tsawg kawg nkaus, tab sis hloov lawv mus rau hauv prism bases. Cov ntaub ntawv ntawm kev txhim kho cov dej khov siv lead ua tau raug tshawb xyuas tag nrho. Tus txheeb ze kev loj hlob ntawm cov ntsiab lus ntawm lub ntsej muag sib txawv nyob ntawm qhov muaj peev xwm los tsim ib qho kev sib koom ua ke hydration. Qhov kub (qis) ntawm cov dej nyob ib puag ncig txiav txim siab qhov degree ntawm branching hauv cov dej khov siv lead ua. Particle loj hlob yog txwv los ntawm tus nqi diffusion ntawm ib tug tsawg degree ntawm supercooling, piv txwv li <2 ° C, ua rau ntau ntawm lawv.

Tab sis txwv los ntawm kev txhim kho kinetics ntawm qib siab ntawm kev nyuaj siab ntawm >4 ° C, ua rau koob loj hlob. Cov duab no zoo ib yam li cov qauv ntawm cov dej khov qhuav (muaj ib qho siv lead ua lattice nrog cov qauv hexagonal), ntau yamCov yam ntxwv ntawm kev txhim kho saum npoo thiab qhov kub thiab txias ntawm ib puag ncig (supercooled) dej, uas yog tom qab lub tiaj tus duab ntawm snowflakes.
Kev tsim cov dej khov hauv cov huab cua muaj txiaj ntsig cuam tshuam rau kev tsim thiab huab cua. Feldspars, pom nyob rau hauv suab puam hmoov av uas nkag mus rau hauv huab cua hauv lab tons hauv ib xyoos, yog cov qub qub. Computer simulations tau pom tias qhov no yog vim lub nucleation ntawm prismatic ice siv lead ua dav hlau ntawm high-zog nto dav hlau.
Qee yam ntsiab lus thiab lattices
Cov tshuaj yaj ywm (tshwj tsis yog me me ntawm helium thiab hydrogen, uas tuaj yeem nkag mus rau hauv cov interstices) tsis tuaj yeem suav nrog hauv Ih qauv ntawm atmospheric siab, tab sis raug yuam tawm mus rau saum npoo los yog amorphous txheej nruab nrab ntawm cov khoom ntawm cov khoom. microcrystalline lub cev. Muaj qee qhov lwm cov ntsiab lus ntawm qhov chaw lattice ntawm cov dej khov qhuav: chaotropic ions xws li NH4 + thiab Cl - uas suav nrog hauv cov kua dej sib zog dua li lwm cov cosmotropic xws li Na + thiab SO42-, yog li tshem tawm lawv tsis tuaj yeem ua tau vim qhov tseeb tias lawv tsim cov yeeb yaj kiab nyias ntawm cov kua uas seem ntawm cov muaju. Qhov no tuaj yeem ua rau hluav taws xob them ntawm qhov chaw vim yog kev sib cais ntawm cov dej saum npoo av ntsuas cov nqi ntxiv (uas tuaj yeem ua rau cov hluav taws xob sib nqus) thiab kev hloov pauv ntawm pH ntawm cov yeeb yaj kiab ua kua, xws li NH 42SO4 ua kua qaub ntau thiab NaCl dhau los yooj yim.
Lawv yog perpendicular rau lub ntsej muagsiv lead ua lattice ntawm cov dej khov qhia cov txheej tom ntej txuas (nrog O atoms dub). Lawv yog tus cwj pwm los ntawm qhov maj mam loj hlob basal nto {0 0 0 1}, qhov twg tsuas yog cov dej molecules sib cais. Kev loj hlob sai sai {1 0 -1 0} nto ntawm lub prism qhov twg khub ntawm cov khoom tshiab txuas tuaj yeem sib txuas nrog ib leeg nrog hydrogen (ib daim ntawv cog lus hydrogen / ob lub molecules ntawm ib lub caij). Lub ntsej muag loj hlob sai tshaj plaws yog {1 1 -2 0} (thib ob prismatic), qhov twg chains ntawm cov khoom txuas tshiab tuaj yeem cuam tshuam nrog ib leeg los ntawm kev sib txuas nrog hydrogen. Ib qho ntawm nws cov saw hlau / cov ntsiab lus molecule yog ib daim ntawv uas tsim cov ridges uas faib thiab txhawb kev hloov mus rau ob sab ntawm lub prism.
Zero point entropy
Can be defined as S 0=k B ˣ Ln (N E0), qhov twg k B yog Boltzmann tas li, NE yog tus naj npawb ntawm kev teeb tsa ntawm lub zog E, thiab E0 yog lub zog qis tshaj. Tus nqi no rau lub entropy ntawm hexagonal ice ntawm xoom Kelvin tsis ua txhaum txoj cai thib peb ntawm thermodynamics "Lub entropy ntawm ib qho zoo tagnrho siv lead ua ntawm qhov tsis muaj tseeb yog xoom", vim tias cov ntsiab lus thiab cov khoom tsis zoo, muaj kev cuam tshuam hydrogen bonding.
Nyob hauv lub cev no, daim ntawv cog lus hydrogen yog random thiab hloov sai heev. Cov qauv no tsis yog qhov sib npaug ntawm lub zog, tab sis txuas mus rau ntau lub xeev uas muaj zog heev, ua raws li "cov cai ntawm dej khov". Zero point entropy yog qhov teeb meem uas yuav nyob twj ywm txawm tias cov khoom tuaj yeem ua kom txias kom meejxoom (0 K=-273, 15 ° C). Tsim kev sim tsis meej pem rau hexagonal ice 3, 41 (± 0, 2) ˣ mol -1 ˣ K -1. Raws li kev xav, nws yuav ua tau los xam qhov xoom entropy ntawm cov dej khov uas paub tias muaj qhov tseeb ntau dua (tsis quav ntsej qhov tsis xws luag thiab qib hluav taws xob sib kis) dua li txiav txim siab nws sim.
Cov kws tshawb fawb thiab lawv txoj haujlwm hauv cheeb tsam no
Can be defined as S 0=k B ˣ Ln (N E0), qhov twg k B yog Boltzmann tas li, NE yog tus naj npawb ntawm kev teeb tsa ntawm lub zog E, thiab E0 yog lub zog qis tshaj. Tus nqi no rau lub entropy ntawm hexagonal ice ntawm xoom Kelvin tsis ua txhaum txoj cai thib peb ntawm thermodynamics "Lub entropy ntawm ib qho zoo tagnrho siv lead ua ntawm qhov tsis muaj tseeb yog xoom", vim tias cov ntsiab lus thiab cov khoom tsis zoo, muaj kev cuam tshuam hydrogen bonding.
Nyob hauv lub cev no, daim ntawv cog lus hydrogen yog random thiab hloov sai heev. Cov qauv no tsis yog qhov sib npaug ntawm lub zog, tab sis txuas mus rau ntau lub xeev uas muaj zog heev, ua raws li "cov cai ntawm dej khov". Zero point entropy yog qhov teeb meem uas yuav nyob twj ywm txawm tias cov khoom tuaj yeem ua kom txias rau qhov tsis muaj tseeb (0 K=-273.15 ° C). Tsim kev sim tsis meej pem rau hexagonal ice 3, 41 (± 0, 2) ˣ mol -1 ˣ K -1. Raws li kev xav, nws yuav ua tau los xam qhov xoom entropy ntawm cov dej khov uas paub tias muaj qhov tseeb ntau dua (tsis quav ntsej qhov tsis xws luag thiab qib hluav taws xob sib kis) dua li txiav txim siab nws sim.

Txawm hais tias qhov kev txiav txim ntawm cov protons hauv cov dej khov ntau tsis tau xaj, qhov saum npoo yuav nyiam qhov kev txiav txim ntawm cov khoom no hauv daim ntawv ntawm cov hlua khi H-atoms thiab O-ib khub (zero entropy nrog xaj hydrogen bonds). Zero point disorder ZPE, J ˣ mol -1 ˣ K -1 thiab lwm tus pom. Los ntawm tag nrho cov saum toj no, nws yog qhov tseeb thiab nkag siab tias hom siv lead ua lattices yog yam ntxwv ntawm cov dej khov.






